|
METHYLENE CHLORIDE
|
|
PRODUCT
IDENTIFICATION
|
| CAS
NO. |
75-09-2 |
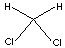
|
| EINECS
NO. |
200-838-9 |
| FORMULA |
CH2Cl2 |
| MOL
WT. |
84.93 |
|
H.S.
CODE
|
2903.12 |
| TOXICITY |
Oral
rat LD50: 1600mg/kg |
| SYNONYMS |
Dichloromethane;
Freon 30; Methylene dichloride; |
| Chlorure De Methylene (French); Chlorocarbon;
Methylene Bichloride; Metylenu Chlorek (Polish); |
| DESCRIPTION |
|
|
CLASSIFICATION
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES
|
| PHYSICAL
STATE |
Clear, colorless liquid |
| MELTING POINT |
-97
C
|
| BOILING
POINT |
40
C |
| SPECIFIC GRAVITY |
1.32 |
| SOLUBILITY
IN WATER |
slightly
soluble |
| pH |
|
| VAPOR DENSITY |
2.9 |
|
AUTOIGNITION
|
556
C
|
|
NFPA
RATINGS
|
Health: 2 Flammability: 1 Reactivity: 0 |
|
REFRACTIVE
INDEX
|
1.4242 |
| FLASH
POINT |
|
| STABILITY |
Stable under ordinary conditions |
|
APPLICATIONS
|
|
Halogenoalkanes, also known as haloalkanes or alkyl halides, are organic
compounds in which one or more hydrogen atoms in an alkane have been replaced by
halogen atoms, fluorine, chlorine, bromine or iodine. In carbon-halogen bond,
halogens have significantly greater electronegativities than carbon except
iodine. In result, this functional group is polarized so that the carbon is
electrophilic and the halogen is nucleophilic. Halogenoalkanes are can be
classified depending on the halogen atom position on the chain of carbon atoms.
The carbon which is attached with the halogen atom is linked up with only one
other alkyl group in primary halogenoalkanes, whereas directly linked up with
two and three other alkyl groups in secondary halogenoalkanes and tertiary
halogenoalkanes respectively. In some case, primary halogenoalkanes are counted
even though there are no alkyl groups attached to the carbon with the halogen on
it. Three characteristics provide important influences on the chemical behavior
of halogenoalkanes, these are electronegativity, covalent bond strength and the
relative stability of the corresponding halide anions. Fluoroalkanes have the
strongest of the carbon-halogen covalent bonds so that they are unreactive. This
is stronger single bond than a carbon-carbon bond. The carbon-chlorine covalent
bond is slightly weaker than a carbon-carbon bond, and the bonds to the other
halogens are weaker. The stability may be estimated from the relative acidities
of the H-X acids. All the hydrohalic acids are very strong, but with small
differences in the direction HCl < HBr < HI, with the exception of HF.
Halogenoarenes, also called haloarene, or aryl Halide, are an organic compound
in which one or more hydrogen atoms in an aromatic ring have been replaced by
halogen atoms. The Haloarenes exhibit many differences compare to haloalkanes in
the method of preparation and their chemical and phisical properties.
Haloalkanes are used in as refrigerants, solvents, blowing agents, aerosol
propellants, fire extinguishing media , and in semiconductor device fabrication.
One of big consumption of halogenoalkanes (properly speaking, halogenoalkenes)
is as a raw material to prepare plastics such as PVC [poly(chloroethene)] from
chloroethene and PTFE [poly(tetrafluoroethene)] from tetrafluoroethene.
Halogenoalkanes and halogenoarenes react with lots of compounds resulting in a
wide range of different target substances. They are useful intermediates in
making other organic compounds.
Methylene chloride
is used in paint and varnish remover formulations, solvent vapor
depressent in aerosol applications, general cleaning solvent and as a foam
blowing agent for flexible polyurethane
foams, |
SALES
SPECIFICATION |
|
APPEARANCE
|
Clear
liquid free from suspended matter
|
| ASSAY |
99.9%
min |
|
SPECIFIC
GRAVITY
|
1.318
- 1.321 |
|
NONVOLATILES
|
10ppm
max |
|
WATER
|
100ppm
max
|
|
COLOR,
APHA
|
10max
|
|
ACIDITY
(HCl)
|
5ppm
max
|
|
FREE
HALOGENS |
Passes
test |
| TRANSPORTATION |
| PACKING |
260kgs
in Drum |
| HAZARD CLASS |
6.1 |
| UN
NO. |
1593 |
| OTHER
INFORMATION |
|
European Hazard
Symbols: XN, Risk Phrases: 40, Safety
Phrases: 23C/24/25/36/37 |
|
CHLORINATED
SOLVENTS
|
|
COMPOUND
|
CAS
#
|
FORMULA
(MOL WT.)
|
BOILING
POINT C
|
DENSITY
|
VAPOR
DENSITY
|
| Methyl chloride |
74-87-3 |
CH3Cl
(50.49) |
-24.2
|
0.915
|
1.74
|
| Methylene chloride |
75-09-2 |
CH2Cl2
(84.93) |
39.8 |
1.3 |
2.9 |
| Chloroacetic Acid |
79-11-8 |
ClCH2COOH
(94.50) |
188
|
1.58
|
3.3
|
| 1,1-Dichloroethene |
75-35-4 |
CH2=CCl2
(96.94) |
31.7 |
1.213 |
3.4
|
| 1,2-Dichloroethylene
(isomer mixture) |
540-59-0 |
ClCH=CHCl
(96.94) |
48 - 60 |
1.3 |
3.4 |
| 1,1-Dichloroethane
|
75-34-3 |
CH3CHCl2
(98.96) |
57.3 |
1.2 |
3.4 |
| Ethylene dichloride |
107-06-2 |
ClCH2CH2Cl
(98.96) |
83.5 |
1.2 |
3.4 |
| Chloroacetic Chloride |
79-04-9 |
ClCH2COCl
(112.94) |
105
|
1.42
|
3.9
|
| 1,2-Dichloropropane |
78-87-5 |
CH3CHClCH2Cl
(112.99) |
96.8 |
1.2 |
3.9 |
| Chloroform
(Trichloromethane) |
67-66-3 |
CHCl3
(119.38) |
61.7 |
1.5 |
4.1 |
| Trichloroethylene |
79-01-6 |
ClCH=CCl2
(131.39) |
86.7 |
1.5 |
4.5 |
| 1,1,1-Trichloroethane
(Methyl Chloroform) |
71-55-6 |
Cl3CCH3
(133.40) |
74.1 |
1.3 |
4.6 |
| 1,1,2-Trichloroethane |
79-00-5 |
ClCH2CHCl2
(133.40) |
113.8 |
1.4 |
4.6 |
| 1,2,3-Trichloropropane |
96-18-4 |
CH2ClCHClCH2Cl
(147.43) |
156 |
1.4 |
5.1 |
| Carbon Tetrachloride |
56-23-5 |
CCl4
(153.82) |
76.7 |
1.6 |
5.3 |
| 1,1,2,2-Tetrachloroethylene |
127-18-4 |
CCl2=CCl2
(165.83) |
121.1 |
1.6 |
5.8 |
| 1,1,2,2-Tetrachloroethane |
79-34-5 |
CHCl2CHCl2
(167.85) |
146.3 |
1.6 |
5.8 |
The
production and use of 1,1,1-trichloroethane and carbon tetrachloride
have been phased out throughout the world because of suspected harm to the earth's ozone layer.
|
|
|
|